Meetings & Events: Penumbra Announces Key Events at SIR 2019

Penumbra Announces Key Events at Society of Interventional Radiology 2019 Meeting
Penumbra, Inc. is pleased to announce key events at the upcoming Society of Interventional Radiology 2019 meeting (SIR 2019) to be held March 23-28 at the Austin Convention Center in Austin, TX. These events include results of a 90-patient embolization study of the company’s Ruby® Coil, the launch of the next generation of innovation for the Indigo® System, a lunch symposium, and in-booth presentations.
James Vogler, M.D.; Dmitri Samoilov, M.D.; and Mark Gemender will share results of a 90-patient study analyzing long-term recanalization data for vessels embolized using Ruby Coils. “Gastroduodenal Artery Recanalization after Transcatheter Occlusion with Ruby Coils for Radioembolization” (abstract #435) will be presented during the Y-DeLiver (Radioembolization III) scientific session (SS45) on Wednesday, March 27 from 3:36–3:45 p.m. in Room 17B.
Attendees will have the opportunity to learn more about Penumbra’s Indigo System, Ruby Coil, POD® System and LANTERN® devices at the following events:
Penumbra Lunch Symposium
Monday, March 25, 12:00–1:00 p.m.
3rd Floor, Room 6B
- Indigo System — Latest Innovation in Power Aspiration for Enhanced Thrombus Removal
James Benenati, M.D., Miami Cardiac & Vascular Institute, FL
- Ruby, POD and Packing Coil: A Complete Platform for Simple to Complex Embolization
Parag Patel, M.D., Medical College of Wisconsin, WI
- PAVM Management: Durable Long-Term Occlusion with POD and Packing Coil
Justin McWilliams, M.D., Ronald Reagan UCLA Medical Center, CA
Eat & Be Educated
Penumbra Booth 621
- Sunday, March 24 · 2:30–3:00 p.m.
The Indigo System, Advanced Technology for Clot Removal in Peripheral Vessels
Kumar Madassery, M.D., Rush University Medical Center, IL
- Monday, March 25 · 2:30–3:00 p.m.
How “Liquid Metal” Packing Coils Have Changed Embolization in My Practice
Bjorn Engstrom, M.D., Abbott Northwestern Hospital, MN
- Tuesday, March 26 · 10:00–10:30 a.m.
Indigo System Is My Frontline Choice for Arterial Thrombus — Tips & Tricks for Maximum Results
Tim Yates, M.D., Mount Sinai Medical Center, FL
- Tuesday, March 26 · 2:30–3:00 p.m.
Embolization Data Deep Dive: The Effect of Dense Metal Packing with Soft, Bare Platinum Coils on Long Term Occlusion
Dr. Dmitri Samoilov, Bon Secours Maryview Medical Center, VA
About Penumbra’s Vascular Products
Penumbra has a growing suite of thrombectomy and embolization products for use in a range of peripheral vascular conditions:

The Indigo System is a next-generation continuous aspiration thrombectomy device designed for the removal of fresh, soft emboli and thrombi from the peripheral venous and arterial systems, and includes catheter sizes ranging from 3.4 F to 8 F. The aspiration lumen is paired with Penumbra ENGINE™, our proprietary continuous vacuum aspiration pump that received 510(k) clearance in March 2018 to evacuate clot. The Indigo System’s proprietary Separator™ technology is designed to enable unobstructed aspiration for the duration of the procedure. The Indigo System received 510(k) clearance in May 2015 to remove thrombi and emboli from the peripheral arterial and venous systems, and it is one of the newest interventional advancements designed to help physicians remove thrombi and emboli associated with ALI and DVTs.
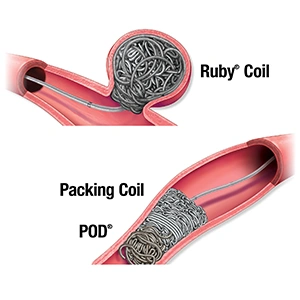
Penumbra’s embolization platform includes Ruby Coil; POD (Penumbra Occlusion Device), designed to occlude peripheral vessels; and Packing Coil, which is uniquely designed to pack very densely behind Ruby and POD to occlude arteries and veins throughout the peripheral vasculature, including aneurysms.

The LANTERN Microcatheter is designed to assist in the delivery of diagnostic agents, such as contrast media, and therapeutic devices, such as occlusion coils, to the peripheral vasculature. It is offered in a variety of lengths and tip shapes.
Risk Information
Caution: Federal (USA) law restricts these devices to sale by or on the order of a physician. Prior to use, please refer to the Instructions for Use for complete product indications, contraindications, warnings, precautions, potential adverse events, and detailed instructions for use.
Indigo Aspiration Catheters and Separators — Indication For Use
As part of the Indigo Aspiration System, the Indigo Aspiration Catheters and Separators are indicated for the removal of fresh, soft emboli and thrombi from vessels of the peripheral arterial and venous systems.
Indigo Aspiration Tubing — Indication For Use
As part of the Indigo Aspiration System, the Indigo Sterile Aspiration Tubing is indicated to connect the Indigo Aspiration Catheters to the Penumbra Aspiration Pump.
Penumbra Aspiration Pump — Indication For Use
The Penumbra Aspiration Pump is indicated as a vacuum source for Penumbra Aspiration Systems.
Contraindications
Not for use in the coronaries or the neurovasculature.
Warnings
• The Indigo Aspiration System should only be used by physicians who have received appropriate training in interventional techniques.
• Do not advance, retract or use any component of the Indigo System against resistance without careful assessment of the cause using fluoroscopy. If the cause cannot be determined, withdraw the device or system as a unit. Unrestrained torquing or forced insertion of the catheter or separator against resistance may result in damage to the device or vessel.
• Do not use the Indigo Aspiration System with a pump other than the Penumbra Aspiration Pump.
Precautions
• The device is intended for single use only. Do not resterilize or reuse. Resterilization and/or reuse may result in ineffective catheter coating lubrication, which may result in high friction and the inability to access the target vasculature location.
• Do not use kinked or damaged devices. Do not use open or damaged packages. Return all damaged devices and packaging to the manufacturer/distributor.
• Use prior to the “Use By” date.
• Use the Indigo Aspiration System in conjunction with fluoroscopic visualization.
• Maintain a constant infusion of appropriate flush solution.
• When performing aspiration, ensure that the Indigo Aspiration Tubing valve is open for only the minimum time needed to remove thrombus. Excessive aspiration or failure to close the Indigo Aspiration Tubing valve when aspiration is complete is not recommended.
• The Indigo Separator is not intended for use as a guidewire. If repositioning of the Indigo Aspiration Catheter is necessary during the revascularization procedure, such repositioning should be performed over an appropriate guidewire using standard microcatheter and guidewire techniques.
• Do not use automated high-pressure contrast injection equipment with the Indigo Aspiration Catheter because it may damage the device.
Potential Adverse Events
Possible complications include, but are not limited to, the following: allergic reaction and anaphylaxis from contrast media; acute occlusion; air embolism; arteriovenous fistula; death; device malfunction; distal embolization; emboli; false aneurysm formation; hematoma or hemorrhage at access site; inability to completely remove thrombus; infection; hemorrhage; ischemia; kidney damage from contrast media; neurological deficits including stroke; vessel spasm, thrombosis, dissection, or perforation; intimal disruption; myocardial infarction; emergent surgery; fibrillation; hypotension; respiratory failure; peripheral thromboembolic events.
Penumbra ENGINE — Indication for Use
The Penumbra ENGINE is indicated as a vacuum source for Penumbra Aspiration Systems.
Contraindications
There are no contraindications.
Warnings/Precautions
• The canister is intended for single use only. Do not reuse. Reuse may result in
canister cracking or vacuum filter blockages, which may result in the inability to
aspirate.
• Do not block bottom air vents. Unit may overheat and shut off or fail to restart if
run for extended periods of time without airflow.
• To avoid the risk of electrical shock, this equipment must only be connected to a
supply mains with protective earth.
• Do not position the Penumbra ENGINE so that it is difficult to remove the power
cord. The means of mains disconnect is to remove the power cord.
• Only use replacement fuse with correct rating (see Table 1 for fuse rating).
• Remove and service the Penumbra ENGINE if liquids or solids have been drawn
into the Penumbra ENGINE.
• Do not use in the presence of a flammable anesthetic mixture with air or nitrous
oxide.
• Do not use in an oxygen rich environment.
• To prevent fire or shock hazard, use a replacement power cord of equal rating.
• Do not re-infuse blood or fluid from the canister back into the patient.
• Do not use petroleum based compounds, acids, caustics, or chlorinated solvents
to clean or lubricate any parts. It will reduce the service life of the Penumbra
ENGINE. Use only water-based solvents for cleaning.
• Use of this equipment adjacent to or stacked with other equipment should be
avoided because it could result in improper operation. If such use is necessary,
this equipment and the other equipment should be observed to verify that they
are operating normally.
• Portable RF communications equipment (including peripherals such as antenna
cables and external antennas) should be used no closer than 12 inches (30cm)
to any part of the Penumbra ENGINE. Otherwise, this could result in degradation
of the performance of this equipment.
• Common emitters (such as RFID emitters, security systems, diathermy
equipment, and portable transmitters) should not be used in close proximity to
the Penumbra ENGINE as they can interfere with and result in degradation of the
performance of the equipment.
• Equipment is not safe for MR use.
• No modification of this equipment is allowed.
Ruby Coil System — Indication for Use
The Ruby Coil System is indicated for arterial and venous embolizations in the peripheral vasculature.
Contraindications
There are no known contraindications.
Warnings
The Ruby Coil System should only be used by physicians who have received appropriate training in interventional techniques.
Precautions
• The device is intended for single use only. Do not resterilize or reuse. Resterilization and/or reuse may compromise the structural integrity of the device or increase the risk of contamination or infection leading to device failure and/or cross-infection and potential patient injury, illness, or death.
• Do not use kinked or damaged devices. Do not use opened or damaged packages. Return all damaged devices and packaging to the manufacturer/distributor.
• Use prior to the “Use By” date.
• Use device in conjunction with fluoroscopic guidance.
• Do not advance or retract the device against resistance without careful assessment of the cause using fluoroscopy.
• Moving or torquing the device against resistance may result in damage to the vessel or device.
• Maintain a constant infusion of an appropriate flush solution.
Potential Adverse Events
Potential complications include but are not limited to:
acute occlusion; air embolism; allergic reaction and anaphylaxis from contrast media; aneurysm rupture; arteriovenous fistula; coagulopathy; coil herniation into parent vessel; death; device malfunction; distal embolization; emboli; embolic stroke and other cerebral ischemic events; false aneurysm formation; hematoma or hemorrhage at access site of entry; incomplete aneurysm occlusion; infection; intima dissection; intracranial hemorrhage; ischemia; myocardial infarction; neurological deficits including stroke; parent artery occlusion; peripheral thromboembolic events; post-embolization syndrome; premature device detachment; recanalization; renal failure; respiratory failure; revascularization; thromboembolic episodes; vessel spasm, thrombosis, dissection, or perforation.
POD System — Indication for Use
For POD Coils with nominal sizes ≤ 6 mm
The POD System is indicated for the embolization of:
• Intracranial aneurysms.
• Other neurovascular abnormalities such as arteriovenous malformations and arteriovenous fistulae.
• Arterial and venous embolizations in the peripheral vasculature.
For POD Coils with nominal sizes > 6 mm
The POD System is indicated for arterial and venous embolizations in the peripheral vasculature.
Contraindications
There are no known contraindications.
Warnings
The POD System should only be used by physicians who have received appropriate training in interventional
techniques.
Precautions
• The device is intended for single use only. Do not resterilize or reuse. Resterilization and/or reuse may compromise the structural integrity of the device or increase the risk of contamination or infection leading to device failure and/or cross-infection and potential patient injury, illness, or death.
• Do not use kinked or damaged devices. Do not use opened or damaged packages. Return all damaged devices and packaging to the manufacturer/distributor.
• Use prior to the “Use By” date.
• Use device in conjunction with fluoroscopic guidance.
• Do not advance or retract the device against resistance without careful assessment of the cause using fluoroscopy. If POD cannot be advanced or retracted, withdraw the device as a unit with the microcatheter.
• Moving or torquing the device against resistance may result in damage to the vessel or device.
• Maintain a constant infusion of an appropriate flush solution.
Potential Adverse Events
Possible complications include, but are not limited to, the following:
acute occlusion; air embolism; allergic reaction and anaphylaxis from contrast media; aneurysm rupture; arteriovenous fistula; coagulopathy; coil herniation into parent vessel; death; device malfunction; distal embolization; emboli; embolic stroke and other cerebral ischemic events; false aneurysm formation; hematoma or hemorrhage at access site of entry; incomplete aneurysm occlusion; infection; intima dissection; intracranial hemorrhage; ischemia; myocardial infarction; neurological deficits including stroke; parent artery occlusion; peripheral thromboembolic events; post-embolization syndrome; premature device detachment; recanalization; renal failure; respiratory failure; revascularization; thromboembolic episodes; vessel spasm, thrombosis, dissection, or perforation.
Penumbra Delivery Microcatheters — Indication for Use
The Penumbra Delivery Microcatheters are intended to assist in the delivery of diagnostic agents, such as contrast media, and therapeutic devices, such as occlusion coils to the peripheral and neuro vasculature.
Contraindications
There are no known contraindications.
Warnings
The Penumbra Delivery Microcatheters should only be used by physicians who have received appropriate training in interventional techniques.
Precautions
• The devices are intended for single use only. Do not resterilize or reuse. Resterilization and/or reuse may result in ineffective catheter coating lubrication, which may result in high friction and the inability to access the target location.
• Do not use kinked or damaged devices. Do not use open or damaged packages. Return all damaged devices and packaging to the manufacturer/distributor.
• Use prior to the “Use By” date.
• Use the Penumbra Delivery Microcatheters in conjunction with fluoroscopic visualization.
• Do not advance or withdraw the Penumbra Delivery Microcatheters against resistance without careful assessment of the cause using fluoroscopy. If the cause cannot be determined, withdraw the device. Moving or torquing the device against resistance may result in damage to the vessel or device.
• Maintain a constant infusion of an appropriate flush solution.
• If flow through the device becomes restricted, do not attempt to clear the lumen by infusion. Remove and replace the device.
Potential Adverse Events
Possible complications include, but are not limited to, the following:
acute occlusion; hematoma or hemorrhage at access site; death; intracranial hemorrhage; hemorrhage; infection (at access site); distal embolization; ischemia (cardiac and/or cerebral); embolus (air, foreign body, thrombus, plaque); aneurysm perforation; false aneurysm formation; neurological deficits including stroke; vessel spasm, thrombosis, dissection, perforation or rupture; air embolism; emboli.
Related Articles
-
 Health
HealthLightning Bolt® 7 Case Study: Rapid Revascularization of Bilateral Renal Artery Occlusion with Computer Assisted Vacuum Thrombectomy (CAVT™)
November 5, 2024 -
 Employee Spotlight
Employee SpotlightEmployee Spotlight: Esteban Zendejas
October 25, 2024 -
 Health
HealthLatest Findings from Penumbra’s STRIKE-PE Study to be Presented at the 2024 Transcatheter Cardiovascular Therapeutics (TCT) Conference
October 24, 2024 -
 Health
HealthGet Out the Clot Campaign Brings Together Leaders and Experts to Improve Patient Care for Venous Thromboembolism
October 10, 2024
