
The First RCT Comparing CAVT™ + Anticoagulation to Anticoagulation Aloneb

Prospective, Randomized Trial

Acute, Intermediate-High Risk PE
Up to 25 Sites
100 Participants
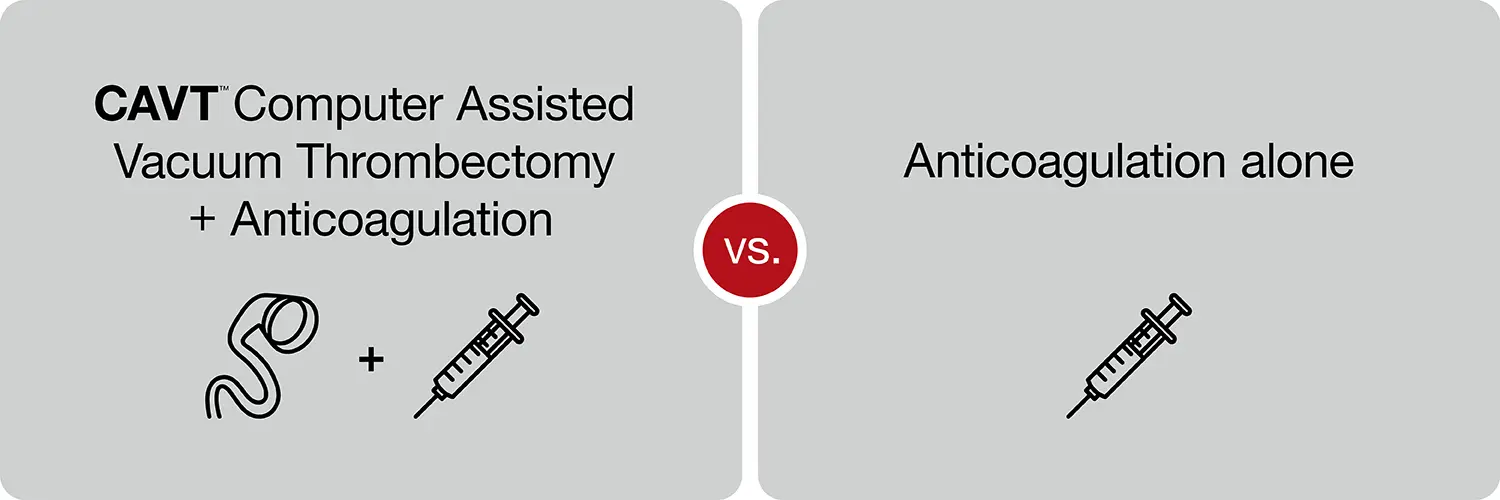
The trial objective is to evaluate the safety and efficacy of treating acute, intermediate-high risk pulmonary embolism with anticoagulation alone versus anticoagulation plus computer assisted vacuum thrombectomy (CAVT) with the Indigo® Aspiration System.
-
- Change in RV/LV ratio assessed by CTPA at 48-hours
- Major adverse events within 7 daysa
- Functional outcomes and quality of life assessments at 90 days
a. Major adverse event is defined as a composite of clinical deterioration requiring escalation of care, PE-related mortality, symptomatic recurrent PE, or major bleeding.
-
STORM-PE patients will be randomized 1:1, divided into the computer assisted vacuum thrombectomy (CAVT) plus anticoagulation arm or the anticoagulation alone arm.
Additional Wearable Device Sub-Study
-
- Symptom onset of 14 days or less
- Acute PE with proximal filling defect confirmed by CTPA
- Intermediate-high risk PE demonstrated by RV/LV ratio and elevated biomarkers
ClinicalTrials.gov Study Site
National Principal Investigators
Massachusetts General Hospital Rachel Rosovsky, MD
Hematology
Mount Sinai Hospital Robert Lookstein, MD
Interventional Radiology
Steering Committee Members
Hadassah Hebrew University Medical Center Ido Weinberg, MD
Vascular Medicine
UCLA Medical Center Richard Channick, MD
Pulmonology
UCLA Medical Center John Moriarty, MD
Interventional Radiology
Columbia University Medical Center Sahil Parikh, MD
Interventional Cardiology
University Medical Center Mainz Stavros Konstantinides, MD
Cardiology
Cedars-Sinai Medical Center Suhail Dohad, MD
Interventional Cardiology
Richard Davis
Patient Representative
b. ONGOING TRIAL. Comparison of two pulmonary embolism treatments – STORM-PE. ClinicalTrials.gov. Updated February 6, 2025. Accessed March 3, 2025. https://clinicaltrials.gov/study/NCT05684796.
